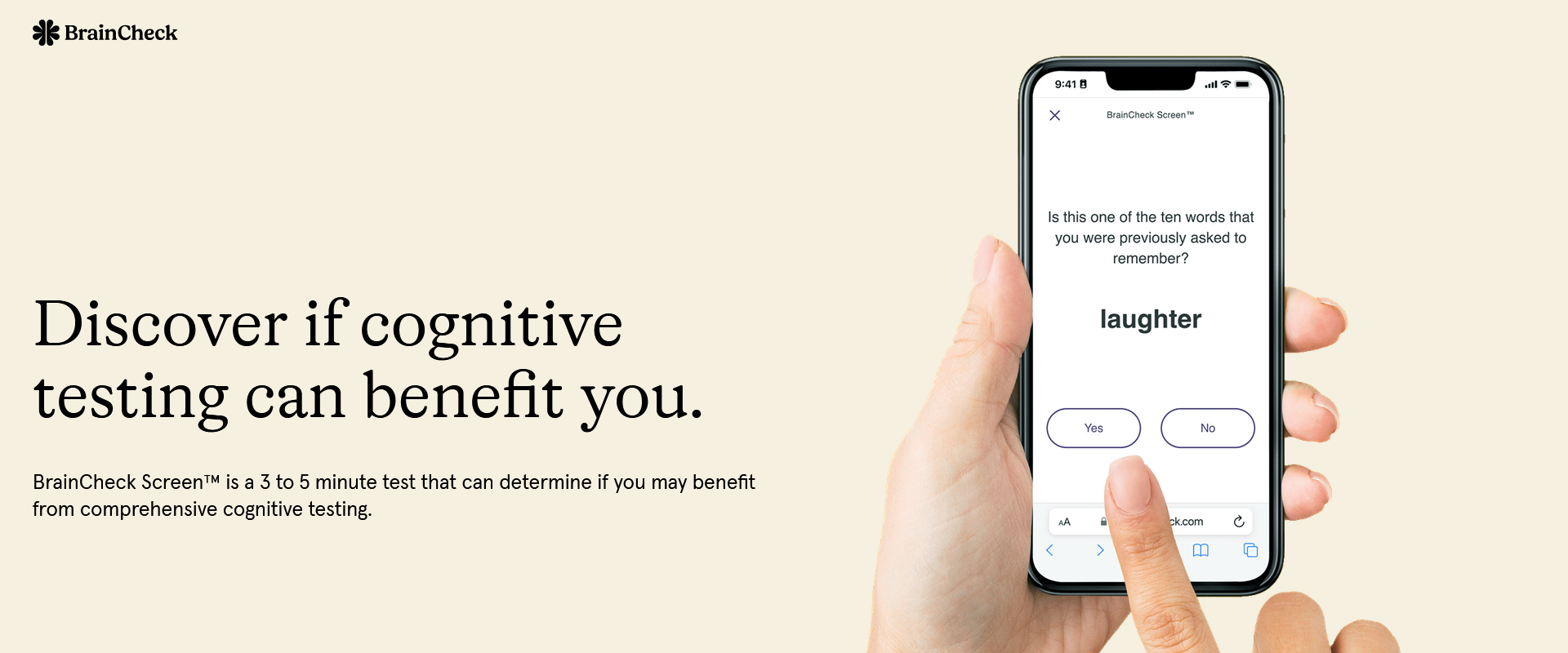
Memory Disorders Clinic
Schedule appointmentClinical Trials
-
Access for ALL in ALS
Status: Not yet recruitingDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Healey Center for ALS Research -
A 12-month Prospective, Randomized, Interventional, Global, Multi-center, Active-controlled Study Comparing Sustained Benefit of Two Treatment Paradigms (AMG334 qm vs. Oral Prophylactics) in Adult Episodic Migraine Patients
-
A Phase 2 Multicenter, Randomized, Double-blind, Placebo-controlled Study of BOTOX® (Botulinum Toxin Type A) Purified Neurotoxin Complex for the Treatment of Upper Limb Essential Tremor
-
A Phase 2, Randomized, Blinded, Placebo-Controlled, Study to Evaluate Safety, Tolerability, Pharmacometrics, and Efficacy of DNTH103 in Adults With Generalized Myasthenia Gravis (MAGIC)
-
A Phase 3 Multicenter 24-Week Open-Label Study to Evaluate the Safety, Tolerability, and Efficacy of Atogepant When Added to OnabotulinumtoxinA (BOTOX) for the Preventive Treatment of Chronic Migraine
-
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Efficacy, Safety, and Tolerability of Atogepant for the Prevention of Chronic Migraine (Progress)
-
A Phase 3, Multi-center, Randomized, Quadruple-blind, Placebo-controlled Study to Assess the Efficacy and Safety of Batoclimab as Induction and Maintenance Therapy in Adult Participants With Generalized Myasthenia Gravis (gMG)
Status: RecruitingDetails: Learn MoreConditions: Myasthenia GravisSponsor: Immunovant Sciences GmbH -
A Phase IIB, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Efficacy and Safety of Intravenous Prasinezumab in Participants With Early Parkinson's Disease
-
A Placebo-Controlled, Double-Blind, Parallel-Group, 18-Month Study With an Open-Label Extension Phase to Confirm Safety and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease
-
A Placebo-Controlled, Double-Blind, Parallel-Group, Bayesian Adaptive Randomization Design and Dose Regimen-finding Study With an Open-Label Extension Phase to Evaluate Safety, Tolerability and Efficacy of BAN2401 in Subjects With Early Alzheimer's Disease
-
A Second Intermediate-Size Expanded Access Protocol (EAP) for Pridopidine in People With Amyotrophic Lateral Sclerosis (Pridopidine EAP 2)
-
An Intermediate Expanded Access Protocol With CNM-Au8 for Amyotrophic Lateral Sclerosis for NIH Grant RFA-NS-23-012
Status: RecruitingDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Clene Nanomedicine -
Long-Term, Observational, Global Registry of Patients With Generalized Myasthenia Gravis Who Have Received Treatment With Complement C5 Inhibition Therapies (C5ITs)
Status: RecruitingDetails: Learn MoreConditions: Myasthenia GravisSponsor: Alexion Pharmaceuticals, Inc. -
A Phase 3, Multicenter, Open-Label 52-Week Extension Study to Evaluate the Long-Term Safety and Tolerability of Oral Atogepant for the Prevention of Migraine in Japanese Participants With Chronic or Episodic Migraine
-
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy, Safety, and Tolerability of AVP-786 (Deudextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Status: Active, not recruitingDetails: Learn MoreConditions: DementiaSponsor: Otsuka Pharmaceutical Development & Commercialization, Inc. -
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Safety and Efficacy of Ravulizumab in Complement-Inhibitor-Naïve Adult Patients With Generalized Myasthenia Gravis
Status: Active, not recruitingDetails: Learn MoreConditions: Myasthenia GravisSponsor: Alexion Pharmaceuticals, Inc. -
A Phase 3, Randomized, Double-blind, Placebo-controlled, Parallel-group, 52-week Study Evaluating the Safety and Efficacy of Simufilam 100 mg Tablets in Subjects With Mild-to-Moderate Alzheimer's Disease
Status: Active, not recruitingDetails: Learn MoreConditions: DementiaSponsor: Cassava Sciences, Inc. -
A Phase II/III Randomized, Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of ABC008 in the Treatment of Subjects With Inclusion Body Myositis
-
A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy, and Safety Study of Gantenerumab in Patients With Early (Prodromal to Mild) Alzheimer's Disease
-
A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Safety, Efficacy, Pharmacokinetics and Pharmacodynamics of ABBV-916 in Subjects With Early Alzheimer's Disease
-
A Second Intermediate Expanded Access Protocol for Amyotrophic Lateral Sclerosis With CNM-Au8
Status: Active, not recruitingDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Clene Nanomedicine -
An Intermediate-Size Expanded Access Protocol for Amyotrophic Lateral Sclerosis with Pridopidine Multicenter Pridopidine EAP
Status: Active, not recruitingDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Healey Center for ALS Research -
An Open-label, Long-term Extension Study to Evaluate the Safety and Tolerability of Simufilam 100 mg Tablets in Participants With Mild to Moderate Alzheimer's Disease
Status: Active, not recruitingDetails: Learn MoreConditions: DementiaSponsor: Cassava Sciences, Inc. -
HEALEY ALS Platform Trial
Status: Active, not recruitingDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Merit E. Cudkowicz, MD -
New IDEAS: Imaging Dementia-Evidence for Amyloid Scanning Study - A Study to Improve Precision in Amyloid PET Coverage and Patient Care
Status: Active, not recruitingDetails: Learn MoreConditions: DementiaSponsor: American College of Radiology -
Phase 3 Multicenter, Randomized, Double-blind, Placebo-controlled Study of BOTOX (Botulinum Toxin Type A) for the Prevention of Migraine in Subjects With Episodic Migraine
-
PLS Natural History Study (PNHS)
Status: Active, not recruitingDetails: Learn MoreConditions: Primary Lateral SclerosisSponsor: ALS Association (ALSA), Mitsubishi Tanabe Pharma, and the Spastic Paraplegia Foundation (SPF), and Mr. David Marren and his family -
A Multicenter, Randomized, Double Blind, Placebo Controlled Parallel Group, Pilot Study to Assess the Efficacy and Safety of Acthar® in Subjects With Relapsing-remitting Multiple Sclerosis
-
A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Crossover Study To Evaluate The Efficacy And Safety Of Zolmitriptan Nasal Spray For The Treatment Of Acute Migraine In Subjects Ages 6 To 11 Years, With An Open-Label Extension
-
A Phase 2a Open-Label, Multi-Center Study to Evaluate the Safety and Tolerability of Multiple Doses of AT-1501 in Adults With ALS
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Anelixis Therapeutics, LLC -
A Phase 3, Multicenter, Long Term, Extension Study of the Safety and Efficacy of AVP-786 (Deuterated [d6] Dextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Status: ClosedDetails: Learn MoreConditions: DementiaSponsor: Otsuka Pharmaceutical Development & Commercialization, Inc. -
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-design Study to Assess the Efficacy, Safety, and Tolerability of AVP-786 (Deudextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Status: ClosedDetails: Learn MoreConditions: DementiaSponsor: Otsuka Pharmaceutical Development & Commercialization, Inc. -
A Phase 3, Open-Label, Multi-Center Trial to Evaluate the Long-Term Safety and Efficacy of Repeat Treatments of DaxibotulinumtoxinA for Injection in Adults With Isolated Cervical Dystonia (ASPEN-OLS)
Status: ClosedDetails: Learn MoreConditions: Movement DisordersSponsor: Revance Therapeutics, Inc. -
A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Group, Multi-Center Trial to Evaluate the Efficacy and Safety of a Single Treatment of DaxibotulinumtoxinA for Injection in Adults With Isolated Cervical Dystonia (ASPEN-1)
Status: ClosedDetails: Learn MoreConditions: Movement DisordersSponsor: Revance Therapeutics, Inc. -
A Phase 4, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to Evaluate the Efficacy and Safety of Erenumab in Adults With Chronic Migraine and Medication Overuse Headache
-
A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial to Evaluate the Safety and Efficacy of AMX0035 Versus Placebo for 48-week Treatment of Adult Patients With Amyotrophic Lateral Sclerosis (ALS)
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Amylyx Pharmaceuticals Inc. -
A Randomized, Double-blind, Double-dummy, Parallel-group Study, Comparing the Efficacy and Safety of Remibrutinib Versus Teriflunomide in Participants With Relapsing Multiple Sclerosis, Followed by Extended Treatment With Open-label Remibrutinib
-
An Expanded Access Protocol of Intravenous Trehalose Injection 90.5 mg/mL Treatment of Patients With Amyotrophic Lateral Sclerosis
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Seelos Therapeutics, Inc. -
Assessment of Safety, Tolerability and Efficacy of LY3002813 in Early Symptomatic Alzheimer's Disease
-
Effect of LY3154207 on Cognition in Mild-to-Moderate Dementia Due to Lewy Body Dementia (LBD) Associated With Idiopathic Parkinson's Disease (PD) or Dementia With Lewy Bodies (DLB)
-
Effects of Oral Levosimendan (ODM-109) on Respiratory Function in Patients With ALS
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Orion Corporation, Orion Pharma -
Efficacy and Safety of Eslicarbazepine Acetate (BIA 2-093) as Adjunctive Therapy for Refractory Partial Seizures in a Double-blind, Randomised, Placebo-controlled, Parallel-group, Multicentre Trial
-
Evaluation of the Safety, Tolerability, Efficacy and Activity of AMX0035, a Fixed Combination of Phenylbutyrate (PB) and Tauroursodeoxycholic Acid (TUDCA), for the Treatment of ALS
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Amylyx Pharmaceuticals Inc. -
Long-term, Prospective,Multinational, Parallel-cohort Study Monitoring Safety in Patients With MS Newly Started With Fingolimod Once Daily or Treated With Another Approved Disease-modifying Therapy
-
Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group Two Year Study to Evaluate the Effect of Subcutaneous RO4909832 on Cognition and Function in Prodromal Alzheimer's Disease With Option for up to an Additional Two Years of Treatment and an Open-Label Extension With Active Study Treatment
-
PROSPECTIVE RANDOMIZED 12-WEEK CONTROLLED STUDY OF VISUAL FIELD CHANGE IN SUBJECTS WITH PARTIAL SEIZURES RECEIVING PREGABALIN OR PLACEBO
Status: ClosedDetails: Learn MoreConditions: Epilepsy/SeizuresSponsor: Pfizer's Upjohn has merged with Mylan to form Viatris Inc. -
Randomized, Double-Blind, Placebo-Controlled, Three-Arm, 12-Month, Safety and Efficacy Study of TRx0237 Monotherapy in Subjects With Alzheimer's Disease Followed by a 12-Month Open-Label Treatment
-
Speech Movement Patterns of Individuals with Amyotrophic Lateral Sclerosis
Status: ClosedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: An NIH-funded project that is being conducted at the University of Texas at Dallas in collaboration with Texas Neurology. The goal of this study is to advance the diagnosis, assessment, and treatment of speech motor impairment due to ALS using novel, computational approaches. -
TRx0237 for the Treatment of Early and Mild-Moderate Alzheimer's Disease: Intermediate-Size Patient Population
-
A 12-month, Randomized, Rater- and Dose-blinded Study to Compare the Efficacy and Safety of Fingolimod 0.25 mg and 0.5 mg Administered Orally Once Daily With Glatiramer Acetate 20 mg Administered Subcutaneously Once Daily in Patients With Relapsing-remitting Multiple Sclerosis
Status: Completed (2018)Details: Learn MoreConditions: Multiple SclerosisSponsor: Novartis Pharmaceuticals -
A 24-month, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group, Efficacy, Safety, Tolerability, Biomarker, and Pharmacokinetic Study of AZD3293 in Early Alzheimer's Disease (The AMARANTH Study)
-
A 26-Week, Double-blind, Randomized, Placebo-controlled, Parallel-group Study to Investigate the Effects of Daily Administration of AC-1204 in Participants With Mild to Moderate Alzheimer's Disease (AD) With an Optional 26-Week Open Label Extension
-
A Double-Blind, Multicenter, Extension Study to Evaluate the Safety and Efficacy of DAC HYP in Subjects With Multiple Sclerosis Who Have Completed Treatment in Study 205MS201 (SELECT)
-
A Double-Blind, Randomized, Placebo-Controlled, Multicenter Study to Assess the Safety and Efficacy and to Determine the Pharmacokinetics of Two Doses of AVP-923 (Dextromethorphan/Quinidine) in the Treatment of Pseudobulbar Affect (PBA) in Patients With Amyotrophic Lateral Sclerosis (ALS) and Multiple Sclerosis (MS)
Status: Completed (2009)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Avanir Pharmaceuticals -
A Multicenter, Double- Blind, Placebo- Controlled Study of Montelukast on Gastrointestinal Tolerability in Patients With Relapsing Forms of Multiple Sclerosis Receiving Tecfidera® (Dimethyl Fumarate) Delayed Release Capsules
-
A Multicenter, Double Blind, Placebo Controlled Study to Assess the Efficacy and Safety of H.P. Acthar® Gel in the Treatment of Subjects With Amyotrophic Lateral Sclerosis
Status: Completed (2019)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Mallinckrodt -
A Multicenter, Double-Blind, Placebo-Controlled, Study to Investigate the Safety and Efficacy of Lithium in Combination With Riluzole in Volunteers With Amyotrophic Lateral Sclerosis (ALS)
Status: Completed (2009)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Massachusetts General Hospital -
A Multi-Center, Open Label Study to Assess the Safety and Tolerability of Riluzole Oral Soluble Film in Subjects With Amyotrophic Lateral Sclerosis Over 12 Weeks of Twice Daily Treatment.
Status: Completed (2018)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Aquestive Therapeutics -
A Multicenter, Open Label, Multiple Dose, Parallel Group Investigation Of The Long-term Safety, Tolerability, Reactogenicity, Pharmacokinetics And Pharmacodynamics Of Aab-001 Administered Subcutaneously In Subjects With Mild To Moderate Alzheimer's Disease
-
A Multicenter, Open-label, Extension Study to Evaluate the Long Term Safety and Efficacy of Daclizumab High Yield Process (DAC HYP) Monotherapy in Subjects With Multiple Sclerosis Who Have Completed Treatment in Study 205MS202 (SELECTION)
-
A Multicenter, Open-Label, Immunogenicity and Safety Study of Avonex® (Interferon Beta-1a) 30 mcg Administered Subcutaneously to Subjects With Relapsing Multiple Sclerosis
-
A Multicenter, Prospective, Randomized, Open-label Study to Compare the Efficacy, Safety, and Tolerability of BOTOX® and Topiramate for Headache Prophylaxis in Adults With Chronic Migraine
-
A Multicenter, Randomized, Open-Label Extension Study to Evaluate the Long-Term Safety and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine With or Without Aura
-
A Multicenter, Retrospective, Observational Study Evaluating Real-world Clinical Outcomes in Relapsing-remitting Multiple Sclerosis Patients Who Transition From Tysabri® (Natalizumab) to Tecfidera® (Dimethyl Fumarate)
-
A Parallel Group, Double-Blind, Randomized, Placebo Controlled Phase 3 Trial to Evaluate the Efficacy and Safety of ALD403 Administered Intravenously in Patients With Chronic Migraine
Status: Completed (2018)Details: Learn MoreConditions: Headache/MigraineSponsor: Alder Biopharmaceuticals, Inc. -
A Phase 2, Multi-Center, Double-Blind, Randomized, Dose-Ranging, Placebo-Controlled Study to Evaluate the Efficacy, Safety and Tolerability of CK-2127107 in Patients With Amyotrophic Lateral Sclerosis (ALS)
Status: Completed (2019)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Cytokinetics -
A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Chronic Migraine Prevention
-
A Phase 2, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention
-
A Phase 2a Randomized Double-blind Placebo Controlled Study to Evaluate the Efficacy and Safety of AMG 301 in Migraine Prevention
-
A Phase 3 Extension, Multicenter, Double-Blind, Long-Term Safety and Tolerability Treatment Trial of Bapineuzumab (AAB-001, ELN115727) in Subjects With Alzheimer's Disease Who Participated in Study ELN115727-301 or Study ELN115727-302.
Status: Completed (2012)Details: Learn MoreConditions: DementiaSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLC -
A Phase 3 Extension, Multicenter, Long Term Safety And Tolerability Trial Of Bapineuzumab (Aab 001, Eln115727) In Subjects With Alzheimer Disease Who Are Apolipoprotein E 4 Carriers And Participated In Study 3133k1-3001-us Or Study 3133k1-3001-ww.
-
A Phase 3 Randomized, Double-blind, Placebo-Controlled Study of the Safety and Effectiveness of Immune Globulin Intravenous (Human), 10% Solution (IGIV, 10%) for the Treatment of Mild to Moderate Alzheimer's Disease
Status: Completed (2013)Details: Learn MoreConditions: DementiaSponsor: Baxalta now part of Shire -
A Phase 3, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (ALS)
Status: CompletedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Cytokinetics -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo Controlled Single Attack Study to Evaluate the Efficacy, Safety, and Tolerability of Oral Ubrogepant in the Acute Treatment of Migraine
-
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study to Assess the Efficacy, Safety, and Tolerability of AVP-786 (Deuterated [d6]-Dextromethorphan Hydrobromide [d6-DM]/Quinidine Sulfate [Q]) for the Treatment of Agitation in Patients With Dementia of the Alzheimer's Type
Status: Completed (2019)Details: Learn MoreConditions: DementiaSponsor: Otsuka Pharmaceutical Development & Commercialization, Inc. -
A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Efficacy And Safety Trial Of Bapineuzumab (Aab-001, Eln115727) In Subjects With Mild to Moderate Alzheimer Disease Who Are Apolipoprotein E 4 Non-carriers
-
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Carriers.
Status: Completed (2012)Details: Learn MoreConditions: DementiaSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLC -
A Phase 3, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Trial of Bapineuzumab (AAB-001, ELN115727) In Patients With Mild to Moderate Alzheimer's Disease Who Are Apolipoprotein E4 Non- Carriers.
Status: Completed (2012)Details: Learn MoreConditions: DementiaSponsor: JANSSEN Alzheimer Immunotherapy Research & Development, LLC -
A Phase 3, Multi-National, Double-Blind, Randomized, Placebo-Controlled, Stratified, Parallel Group, Study to Evaluate the Safety, Tolerability and Efficacy of Tirasemtiv in Patients With Amyotrophic Lateral Sclerosis (ALS)
Status: Completed (2017)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Cytokinetics -
A Phase 3, Open-Label Extension Study of Tirasemtiv for Patients With Amyotrophic Lateral Sclerosis (ALS) Who Completed VITALITY-ALS (CY 4031)
Status: Completed (2018)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Cytokinetics -
A Phase 3, Randomized, Double-Blind Study to Evaluate the Safety and Efficacy of Elvitegravir/Emtricitabine/Tenofovir Disoproxil Fumarate/GS-9350 Versus Ritonavir-Boosted Atazanavir Plus Emtricitabine/Tenofovir Disoproxil Fumarate in HIV-1 Infected, Antiretroviral Treatment-Naive Adults
Status: Completed (2014)Details: Learn MoreConditions: Infectious DiseasesSponsor: Gilead Sciences -
A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Efficacy and Safety of AMG 334 in Migraine Prevention
-
A Phase II Randomized, Double-Blinded, Placebo-Controlled, Multi-Center Study of Subcutaneous Daclizumab in Patients With Active, Relapsing Forms of Multiple Sclerosis
Status: Completed (2006)Details: Learn MoreConditions: Multiple SclerosisSponsor: PDL BioPharma, Inc. -
A Phase IIb, Multi-National, Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Safety, Tolerability and Efficacy of CK-2017357 in Patients With Amyotrophic Lateral Sclerosis (ALS) (BENEFIT-ALS)
Status: Completed (2014)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Cytokinetics -
A Phase III Randomized, Placebo-Controlled Clinical Trial to Study the Safety and Efficacy of Suvorexant (MK-4305) for the Treatment of Insomnia in Subjects With Alzheimer's Disease
-
A Phase Iii, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel Group, Efficacy And Safety Trial Of Bapineuzumab (Aab 001, Eln115727) In Subjects With Mild To Moderate Alzheimer Disease Who Are Apolipoprotein E 4 Carriers
-
A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Efficacy and Safety Study of Crenezumab in Patients With Prodromal to Mild Alzheimer's Disease
-
A Randomized, Double-Blind, Placebo-Controlled, Multi-Center Study of the Safety and Efficacy of Dexpramipexole in Subjects With Amyotrophic Lateral Sclerosis
Status: Completed (2012)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Knopp Biosciences -
A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Multicenter Study to Determine the Safety and Efficacy of Natalizumab in Subjects With Relapsing-Remitting Multiple Sclerosis
-
A Randomized, Placebo Controlled, Parallel-Group, Double Blind Efficacy and Safety Trial of MK-8931 With a Long Term Double-Blind Extension in Subjects With Mild to Moderate Alzheimer's Disease (Protocol No. MK-8931-017-10)(Also Known as SCH 900931, P07738)
-
A Seamless Phase IIa/IIb, Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Trial to Evaluate the Efficacy and Safety of MK-7622 as an Adjunctive Therapy for Symptomatic Treatment in Subjects With Alzheimer's Disease
-
A Study to Explore the Safety and Tolerability of Acthar in Patients With Amyotrophic Lateral Sclerosis
Status: Completed (2014)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Mallinckrodt -
ALS COSMOS (ALS Cohort Study of Multicenter Oxidative Stress)
Status: Completed (2016)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: National Institute of Health (NIH) -
ALS Patient Care Database
-
An Extension Study to Evaluate the Long-Term Safety, Tolerability and Efficacy of Dalfampridine Extended-Release Tablets. for the Treatment of Chronic Post-Ischemic Stroke Walking Deficits .
-
An Open-label Extension (OLE) Study to Assess the Long-term Safety and Efficacy of AMG 334
-
An Open-Label Study to Evaluate the Safety of NP101, a Sumatriptan Iontophoretic Transdermal Patch, in the Treatment of Acute Migraine Over 12 Months
-
An Open-Label, Multicenter, Extension Study to Evaluate the Long-Term Safety and Efficacy of Dexpramipexole (BIIB050) in Subjects With Amyotrophic Lateral Sclerosis
Status: Completed (2013)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Knopp Biosciences -
An Open-label, Multinational, Multicenter, Follow-up Study to Evaluate the Long-term Safety and Efficacy of Brivaracetam Used at a Flexible Dose up to a Maximum of 200 mg/Day in Subjects Aged 16 Years or Older Suffering From Epilepsy.
-
Analysis of Transcriptional Changes in Blood Samples from Patients with Neurologic Diseases
Status: Completed (2013)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Baylor Research Institute and Neurologix Foundation -
Answer ALS -Creation of a Large Bio-repository of iPS Cells, Cell Lines, and Bio-fluid Samples, Combined With Clinical Information to Rapidly Advance Therapeutics That Could Treat ALS
Status: Completed (2020)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Johns Hopkins University -
Biogen Idec Evidera ALS Study: Qualitative Investigation of Signs and Related Functional Decline in Patients with Amyotrophic Lateral Sclerosis(ALS)
Status: Completed (2015)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Biogen Idec, Inc. -
Centers for Disease Control IRB - Protocol 5923.0 – “Proposal for the State and Metropolitan Area-Based ALS Surveillance”
-
Clinical Trial Ceftriaxone in Subjects With Amyotrophic Lateral Sclerosis (ALS)
Status: Completed (2012)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Massachusetts General Hospital -
Collection of Blood Samples for DNA Analysis in Motor Neuron Diseases
Status: Completed (2013)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: National Institute of Neurological Disorders and Stroke (NINDS) -
Double-Blind, Placebo-Controlled, Parallel-Group Study to Evaluate the Safety and Efficacy of Two Doses of Oral Dalfampridine Extended Release Tablets (5 mg and 10 mg Twice Daily) in Patients With Multiple Sclerosis
Status: Completed (2012)Details: Learn MoreConditions: Multiple SclerosisSponsor: Acorda Therapeutics -
Double-Blind, Randomized, Historical Control Study of the Safety and Efficacy of Eslicarbazepine Acetate Monotherapy in Subjects With Partial Epilepsy Not Well Controlled by Current Antiepileptic Drugs
Status: Completed (2013)Details: Learn MoreConditions: Epilepsy/SeizuresSponsor: Sumitomo Pharma America, Inc. -
Effect of Passive Immunization on the Progression of Mild Alzheimer's Disease: Solanezumab (LY2062430) Versus Placebo
-
Effect of Septal Closure of Atrial PFO on Events of Migraine With Premere: ESCAPE Migraine Trial
Status: Completed (2012)Details: Learn MoreConditions: Headache/MigraineSponsor: Abbott Medical Devices -
Long Term Eslicarbazepine Acetate Extension Study
Status: Completed (2017)Details: Learn MoreConditions: Epilepsy/SeizuresSponsor: Sumitomo Pharma America, Inc. -
Multicenter, Double-Blind, Placebo-Controlled, Dose-Ranging Study to Determine the Safety and Efficacy of Daclizumab HYP (DAC HYP) as a Monotherapy Treatment in Subjects With Relapsing-Remitting Multiple Sclerosis
-
NINDS Clinical Research Collaboration Chronic Migraine Treatment Trial
-
Northeast ALS (NEALS) Consortium: The Upper Motor Neuron Disease (UMND) Pilot Registry
-
Open-Label Study to Evaluate Safety, Tolerability and Pharmacokinetics of Multiple Doses of BHV-0223 in Subjects With Amyotrophic Lateral Sclerosis
Status: Completed (2018)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Biohaven Pharmaceuticals, Inc. -
Patient Assisted Intervention for Neuropathy: Comparison of Treatment in Real Life Situations (PAIN-CONTRoLS)
Status: Completed (2017)Details: Learn MoreConditions: NeuropathiesSponsor: University of Kansas Medical Center -
Safety and Efficacy of ADS-5102 (Amantadine HCl) Extended Release Capsules in Patients With Multiple Sclerosis and Walking Impairment
Status: Completed (2016)Details: Learn MoreConditions: Multiple SclerosisSponsor: Adamas Pharmaceuticals, Inc. -
Safety Study of Oral Edaravone Administered in Subjects With ALS
Status: CompletedDetails: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Mitsubishi Tanabe Pharma America Inc. -
Texas Department of State Health Services IRB – # 10-036 – “Texas Amyotrophic Lateral (ALS) Surveillance Activity”
-
This is a Multi-center, Double-blind, Three Arm, Parallel Group, Placebo-controlled, Randomized Study Designed to Evaluate the Efficacy, Safety and Tolerability of Dalfampridine.
-
TIV (Tracheostomy with invasive ventilation) for Patients with ALS in Japan and the USA: A Comparitive Study
Status: Completed (2011)Details: Learn MoreConditions: Amyotrophic Lateral SclerosisSponsor: Columbia University and the MDA